The worksheet periodic table answer key stands as an indispensable guide in the exploration of chemistry’s foundational framework. This comprehensive resource empowers educators and students alike to decipher the intricacies of the periodic table, unlocking its secrets and revealing its profound impact on our understanding of the chemical world.
Within this meticulously crafted document, learners will embark on a journey through the periodic table’s rich tapestry of elements, uncovering their unique properties and behaviors. Armed with this invaluable tool, they will gain a deeper appreciation for the periodic table’s role in organizing and classifying the building blocks of matter, laying the groundwork for a comprehensive understanding of chemistry.
Worksheet: Periodic Table
A periodic table worksheet is a learning tool used in chemistry education to reinforce understanding of the periodic table and its elements. Worksheets may include various activities, such as filling in missing elements, identifying element properties, or solving problems related to the periodic table.
Types of Periodic Table Worksheets
There are several types of periodic table worksheets, each designed for specific educational purposes:
- Blank Periodic Table:A worksheet with an empty periodic table for students to fill in element symbols, atomic numbers, and other information.
- Element Properties Identification:A worksheet that provides a list of element properties (e.g., atomic mass, electron configuration) and asks students to identify the corresponding elements on the periodic table.
- Periodic Table Trends:A worksheet that guides students in identifying trends in element properties across the periodic table, such as atomic radius, ionization energy, and electronegativity.
- Periodic Table Problem Solving:A worksheet that presents problems related to the periodic table, such as predicting element reactivity or determining the products of chemical reactions.
Benefits of Using Periodic Table Worksheets
Periodic table worksheets offer numerous benefits for students:
- Reinforce Understanding:By completing worksheets, students actively engage with the periodic table and reinforce their understanding of element properties and relationships.
- Develop Problem-Solving Skills:Problem-solving worksheets challenge students to apply their knowledge of the periodic table to real-world scenarios.
- Improve Critical Thinking:Worksheets encourage students to analyze and interpret data, promoting critical thinking skills.
- Prepare for Assessments:Worksheets can serve as practice exercises for tests and assessments, helping students prepare for summative evaluations.
Answer Key: Periodic Table
An answer key is an essential component of a periodic table worksheet. It provides the correct answers to the questions or exercises on the worksheet, allowing students to check their work and identify areas where they need improvement.
There are different types of answer keys for periodic table worksheets. Some answer keys simply provide the correct answers to the questions, while others provide more detailed explanations and worked-out solutions.
Benefits of Using an Answer Key
- Provides immediate feedback:An answer key allows students to check their work immediately, providing them with feedback on their understanding of the material.
- Identifies areas for improvement:By comparing their answers to the answer key, students can identify areas where they need to improve their understanding.
- Saves time:An answer key saves teachers time by providing them with a quick and easy way to grade student work.
- Encourages independent learning:An answer key can encourage independent learning by allowing students to check their work and identify areas where they need additional support.
Key Concepts
A periodic table worksheet should cover the following key concepts:
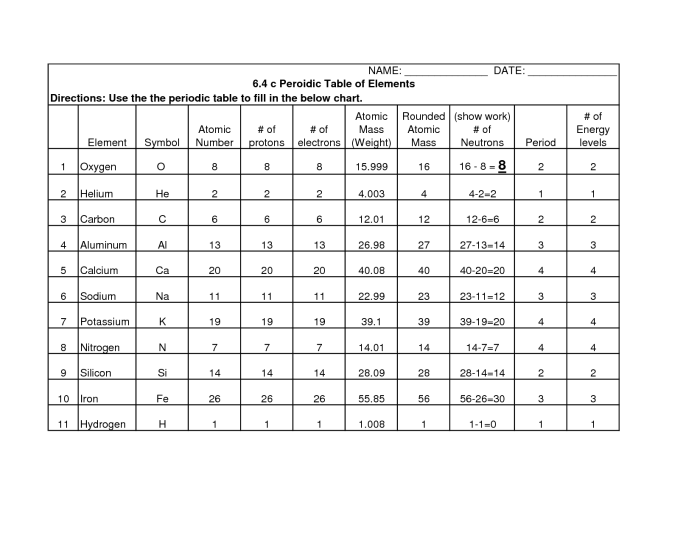
Atomic Number:The number of protons in the nucleus of an atom. It determines the element’s position on the periodic table and its chemical properties.
Atomic Mass:The weighted average mass of all the isotopes of an element. It is used to calculate the molar mass of an element.
Electron Configuration:The arrangement of electrons in the orbitals of an atom. It determines the element’s chemical reactivity.
Periodic Trends:The regular changes in properties of elements as you move across or down the periodic table. These trends include atomic radius, ionization energy, and electronegativity.
Group and Period:The vertical and horizontal rows on the periodic table, respectively. Elements in the same group have similar chemical properties, while elements in the same period have the same number of electron shells.
Importance of Key Concepts
Understanding these key concepts is essential for understanding the periodic table and its applications in chemistry.
The atomic number determines the element’s identity and its position on the periodic table. The atomic mass is used to calculate the molar mass of an element, which is essential for stoichiometric calculations.
The electron configuration determines the element’s chemical reactivity. Elements with similar electron configurations have similar chemical properties.
Periodic trends can be used to predict the properties of an element based on its position on the periodic table. For example, elements in the same group tend to have similar ionization energies.
Groups and periods help organize the elements on the periodic table and make it easier to find information about specific elements.
Incorporation into Worksheet
A periodic table worksheet can incorporate these key concepts in several ways:
- Identifying the atomic number, atomic mass, and electron configuration of specific elements.
- Explaining the periodic trends for a particular property, such as atomic radius or ionization energy.
- Matching elements to their group and period based on their properties.
- Using the periodic table to predict the properties of an unknown element.
Educational Value
Periodic table worksheets are valuable educational tools for teaching chemistry. They provide students with a visual representation of the elements and their properties, making it easier for them to understand the periodic trends and relationships between elements.Periodic table worksheets can be used to teach students about a variety of chemistry concepts, including:
- The arrangement of elements in the periodic table
- The periodic trends, such as atomic radius, ionization energy, and electronegativity
- The chemical properties of elements
- The relationship between the structure of an atom and its properties
- The uses of elements in everyday life
Periodic table worksheets can be used in a variety of educational settings, including:
- As a teaching tool in the classroom
- As a review tool for students
- As a homework assignment
- As a quiz or test
Periodic table worksheets are a versatile and effective way to teach students about chemistry. They can be used to teach a variety of concepts, and they can be used in a variety of educational settings.
HTML Table Structure: Periodic Table
HTML tables provide a structured and organized way to display data, making them ideal for presenting the periodic table. Tables allow for easy navigation and quick access to information about each element.
To create an HTML table, use the
| tag. The following code demonstrates a basic HTML table structure for the periodic table:
“`html
“` This table structure can be further styled using CSS to improve its appearance and readability. For example, the following CSS rules can be used to style the periodic table: “`csstable border-collapse: collapse; border: 1px solid black;th, td border: 1px solid black; padding: 5px;“` By using an HTML table to display the periodic table, we can create a visually appealing and informative resource that is easy to navigate and use. Bullet Point Organization: Periodic Table: Worksheet Periodic Table Answer KeyOrganizing information about the periodic table using bullet points enhances clarity and conciseness. Bullet points present key points in a structured and visually appealing format, making it easier for readers to grasp and retain information. Bullet points can be used to organize various aspects of the periodic table, such as: Periodic Table Groups
Periodic Table Periods
Periodic Table Properties
Real-World ApplicationsThe periodic table is a powerful tool that has numerous real-world applications. It can be used to solve problems in various fields, including chemistry, physics, biology, and geology. One of the most important applications of the periodic table is in the field of chemistry. The periodic table can be used to predict the chemical properties of elements. This information can be used to design new materials, develop new drugs, and understand the behavior of chemicals in the environment. Applications in IndustryThe periodic table is also used in a variety of industries. For example, in the steel industry, the periodic table is used to select the appropriate alloys for different applications. In the electronics industry, the periodic table is used to design semiconductors and other electronic components. Applications in MedicineThe periodic table is also used in medicine. For example, the periodic table can be used to design new drugs and understand the mechanisms of disease. The periodic table can also be used to develop new medical imaging techniques. Historical Development: Periodic TableThe periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configurations, and recurring chemical properties. Its development has been a gradual process, with contributions from numerous scientists over several centuries. Johann Wolfgang Döbereiner
Alexandre-Emile Béguyer de Chancourtois
John Newlands
Lothar Meyer and Dmitri Mendeleev
Impact of the Periodic TableThe periodic table has had a profound impact on the development of chemistry. It provides a systematic way to organize and understand the chemical elements and their properties. It has also led to the discovery of new elements and the development of new theories in chemistry. Modern ApplicationsThe periodic table continues to play a vital role in modern scientific research and technological advancements. It provides a systematic framework for understanding the properties of elements and predicting their behavior in various chemical reactions. In cutting-edge research, the periodic table is used to guide the design and synthesis of new materials with tailored properties. For example, scientists can use the periodic table to identify elements with specific electronic configurations or atomic radii that are suitable for creating materials with desired optical, electronic, or magnetic properties. Drug DevelopmentThe periodic table is also a valuable tool in drug development. By understanding the relationship between the structure and properties of elements, researchers can design drugs that target specific biological molecules or pathways. For example, the discovery of platinum-based drugs revolutionized cancer treatment, and the development of lithium-based drugs has provided effective treatments for bipolar disorder. NanotechnologyThe periodic table is essential in the field of nanotechnology, where scientists manipulate matter at the atomic and molecular scale. By understanding the properties of different elements, researchers can design and synthesize nanoparticles with specific shapes, sizes, and compositions. These nanoparticles have a wide range of applications, including drug delivery, imaging, and energy storage. Environmental ScienceThe periodic table also plays a crucial role in environmental science. It helps scientists understand the behavior and fate of pollutants in the environment. For example, the periodic table can be used to predict the solubility and toxicity of heavy metals, which are common environmental contaminants. Common MisconceptionsDespite its widespread use, the periodic table is not without its misconceptions. These misconceptions can lead to misunderstandings about the elements and their properties. One common misconception is that the periodic table is a static structure. In reality, the periodic table is constantly being updated as new elements are discovered and new information is learned about existing elements. Misconception 1: The Periodic Table is a Static Structure
Misconception 2: The Periodic Table is Only for Chemists, Worksheet periodic table answer key
Misconception 3: The Periodic Table is Too Complex to Understand
Query ResolutionWhat is the purpose of a periodic table worksheet? Periodic table worksheets are designed to enhance students’ understanding of the periodic table’s organization, element properties, and chemical trends. How can I use a periodic table answer key effectively? An answer key provides accurate solutions to worksheet questions, allowing students to self-assess their understanding and identify areas for improvement. What are some common misconceptions about the periodic table? Common misconceptions include believing that the periodic table is static or that all elements in a group have identical properties. |